Establishing a Carrot Cavity Spot Nursery at Washington State University
By Lindsey du Toit and Michael Derie, Washington State University
Why Set up a Cavity Spot Nursery?
Cavity spot occurs in almost all regions of carrot production. This disease is listed by the California Fresh Carrot Advisory Board (CFCAB) as one of the primary concerns for carrot growers in California, who produce 70 percent of the fresh market carrots in the U.S. The pathogens associated most commonly with cavity spot are Pythium violae and P. sulcatum, but other species of Pythium can cause this disease.
These are not true fungi, but water molds (oomycetes). These pathogens survive in soils where they produce two kinds of spores: long-lived, sexual spores called oospores, and short-lived, swimming spores called zoospores. The oospores are triggered to germinate by chemicals that roots of plants exude into the soil. The pathogens are most active and usually cause the most damage in cool, moist soil conditions.
Cavity spot seldom causes a reduction in overall carrot root yield (weight or size of roots). However, the disease can have a very significant economic impact for growers because the shallow, sunken lesions caused by the pathogens on the surface of roots make the roots unmarketable for fresh or processing markets (Fig. 1). The lesions on roots start as very small (a few millimeters in diameter), sunken areas that can increase to more than 1 inch in length.

Figure 1. These severe symptoms of cavity spot on carrot roots are caused by Pythium sulcatum. Photo courtesy Alex Batson, WSU graduate student
Pythium species usually infect carrot roots within four to six weeks after planting, but infection can continue as long as roots remain in the soil. Cavity spot usually increases the longer carrot roots are left in the soil. The disease will even continue to develop on roots that have been harvested and placed in storage. The lesions or cavities on the roots can be invaded by secondary microorganisms, including bacteria. This can cause the cavities to become discolored, particularly during heating/blanching of carrots being processed (Fig. 2).
Growers often struggle to manage cavity spot using cultural practices and fungicides. Recommendations to control cavity spot include avoiding fields with a history of the disease, using a crop rotation of at least three to four years between carrot crops, not using high rates of nitrogen fertilization, planting in fields with good drainage that is less favorable for the swimming spore stage, not planting in cold soils and harvesting roots in a timely manner to limit development of cavity spot. Some fungicides can be highly effective against cavity spot, such as metalaxyl or mefenoxam (e.g. Ridomil). Unfortunately, the pathogens that cause cavity spot are notorious for developing resistance to fungicides like mefenoxam, which severely limits the ability for growers to control cavity spot using fungicides.
Given the difficulty of managing cavity spot, many seed companies are trying to breed for resistance to the disease, but the process is not easy and progress has been slow. There are differences in susceptibility to cavity spot among commercial cultivars, but there are no cultivars that are completely resistant to the disease. Carrot growers in some states continue to fund Phil Simon, USDA-ARS carrot breeder based in Madison, Wisconsin, to develop cultivars with better resistance to cavity spot. Simon has been collaborating with Mary Ruth McDonald, plant pathologist at the University of Guelph in Ontario, Canada, in efforts to breed for resistance to cavity spot. McDonald has established a cavity spot nursery in a muck soil in Ontario that is naturally infected with cavity spot pathogens.
In most regions of carrot production in the U.S., the soils are sandy/mineral, so growers in California have been wanting to create a cavity spot nursery in a field with mineral soil. In 2019, the CFCAB provided funding to Lindsey du Toit’s program at Washington State University (WSU) to establish a cavity spot nursery at the WSU Mount Vernon Northwestern Washington Research & Extension Center (NWREC) in Mount Vernon, Washington, to complement the muck cavity spot nursery in Ontario and provide further support for Simon’s efforts at breeding for resistance to this disease.
How to Establish a Cavity Spot Nursery
To establish the cavity spot pathogens in the field site in Mount Vernon, a 1-acre field was fumigated with metam sodium in fall 2018 to kill microorganisms in the soil that might compete with cavity spot pathogens. The field was then inoculated with P. violae and P. sulcatum three weeks later. The inoculum was produced by growing the pathogens on vermiculite moistened with V8 juice (Yes, Pythium species like V8 juice as much as some people like Bloody Marys!) in mushroom bags. About 250 gallons of inoculum were spread over the field using a tractor mounted, PTO-driven, Viton sling spreader (Fig. 3). The inoculum was incorporated into the soil by rototilling. The field was inoculated again with 266 gallons of inoculum of P. violae and P. sulcatum in April 2019.

Figure 3. Inoculum of Pythium sulcatum and Pythium violae was applied to a field at Washington State University using a spreader, and then incorporated by rototilling, in fall 2018, spring 2019 and fall 2019 to help establish the WSU Carrot Cavity Spot Nursery.
On May 3, 2019, 219 carrot breeding lines and 12 commercial carrot cultivars were planted in replicate blocks in the field. The cultivar Atomic Red, which is highly susceptible to cavity spot, was planted throughout the trial as a susceptible check to assess the uniformity in cavity spot pressure across the field. Each plot was a single row, 10 feet long. Rows were spaced 20 inches. The seed for each plot was planted using a Wintersteiger cone push planter set to distribute 100 seeds in each plot. The trial was irrigated 19 times between May and early September to promote carrot root growth and development of cavity spot.
In September 2019, each plot was rated for the percentage of plants that had bolted (started flowering) and for average height of the plants. In October 2019, the roots in each plot were undercut, dug manually, washed and rated for the percentage of roots in each plot with symptoms of cavity spot and the severity of symptoms on each root based on the size of the largest lesion on each root (Fig. 4, Fig. 5).

Figure 4. Researchers undercut, dig and wash carrot roots in the WSU Carrot Cavity Spot Nursery in 2019 to evaluate replicated plots of 231 Plant Introductions, breeding lines and cultivars for resistance to cavity spot.
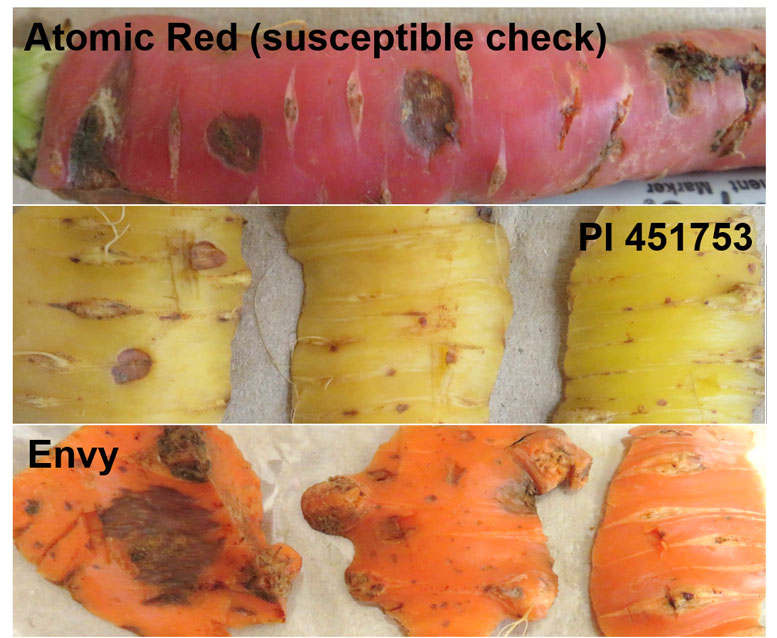
Figure 5. A variation in size of cavity spot lesions was observed on the roots of Atomic Red, Plant Introduction 451752 and Envy in the WSU Carrot Cavity Spot Nursery in 2019.
Success at Establishing the WSU Carrot Cavity Spot Nursery
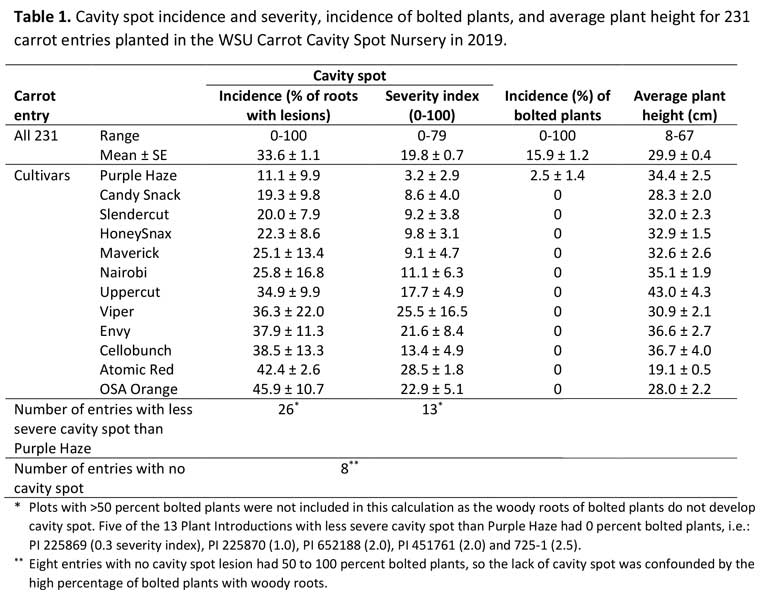
Cavity spot symptoms were observed on all but eight of the 231 carrot lines evaluated, including all 12 named cultivars. Isolations from root lesions confirmed the symptoms were cavity spot, as Pythium was isolated from all of the lesions tested. There was a wide range in incidence and severity of cavity spot among the 231 lines, which demonstrated success at establishing the WSU Carrot Cavity Spot Nursery. For the 231 carrot entries evaluated, the percentage of roots with cavity spot ranged from 0 to 100 (average of 33.6 percent), and the severity ranged from 0 to 79 (average of 19.8) (Table 1).
Although cavity spot symptoms were observed on the vast majority of the carrot lines evaluated, for the eight entries with no cavity spot, 50 to 100 percent of the plants had bolted. When plants bolt, the roots turn “woody” and do not show symptoms of cavity spot, as demonstrated by the negative correlation between the percentage of plants bolted and both the incidence and severity of cavity spot. Therefore, the eight lines with a high percentage of bolting and no cavity spot were not included in the data analysis for cavity spot.
Of the 12 carrot cultivars evaluated, the incidence and severity of cavity spot was least on Purple Haze. Based on trials in Ontario and other sites, Purple Haze is partially resistant to cavity spot. In the WSU Cavity Spot Nursery, 11.1 percent of Purple Haze roots had cavity spot lesions, with a mean severity of 3.2 (Table 1). In contrast, the percentage of roots with cavity spot was greatest on Atomic Red, the susceptible control cultivar (42.4 percent of roots had cavity spot, with an average severity of 28.5). Of the other breeding lines and plant introductions (PIs), 26 had fewer roots with cavity spot than Purple Haze, and 13 had less severe cavity spot symptoms than Purple Haze, the partially resistant cultivar. Some of these 13 lines had a lot of bolted plants, which confounded cavity spot ratings, but five of the 13 had no bolted plants. This included PI 225869 (0.3 severity of cavity spot), PI 225870 (1), PI 652188 (2), PI 451761 (2) and 725-1 (2.5). These lines might be valuable sources of resistance to cavity spot. The entries will be evaluated again in 2020 in the WSU Carrot Cavity Spot Nursery to determine the consistency in response of the cultivars. The percentage of roots with cavity spot was significantly correlated with the severity of cavity spot across all the lines tested (r = 0.9154 at P< 0.0001).
The percentage of plants that bolted ranged from 0 to 100 (average of 15.9 percent) and was particularly severe for some PIs. None of the named cultivars had bolted plants, except for Purple Haze, which only had 2.5 percent bolted plants. Carrot rust fly pressure was also quite severe in the trial.
What’s Next?
After rating the carrot roots in October, the roots were spread out on the field and disked into the soil to increase cavity spot pressure for planting another trial in 2020. In addition, a third batch of inoculum of P. sulcatum and P. violae was produced on vermiculite with V8 juice, applied to the field in October 2019 and incorporated into the soil. The field will be inoculated again in spring 2020 to continue building cavity spot disease pressure for this nursery. This nursery will support the development of carrot cultivars with improved resistance to cavity spot.
The 2020 WSU Carrot Cavity Spot Nursery trial will be included in the field tour as part of the 40th International Carrot Conference on Oct. 5-6, 2020, at the WSU Mount Vernon NWREC. For more details on that conference, visit www.internationalcarrots.org.